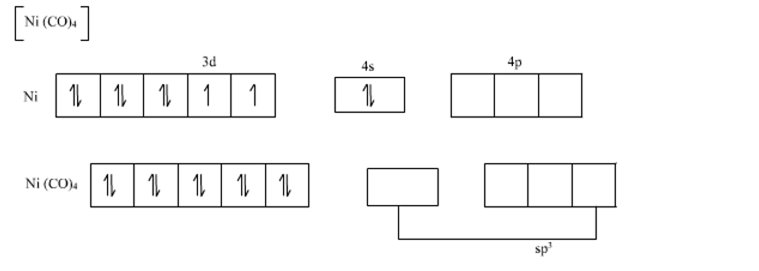
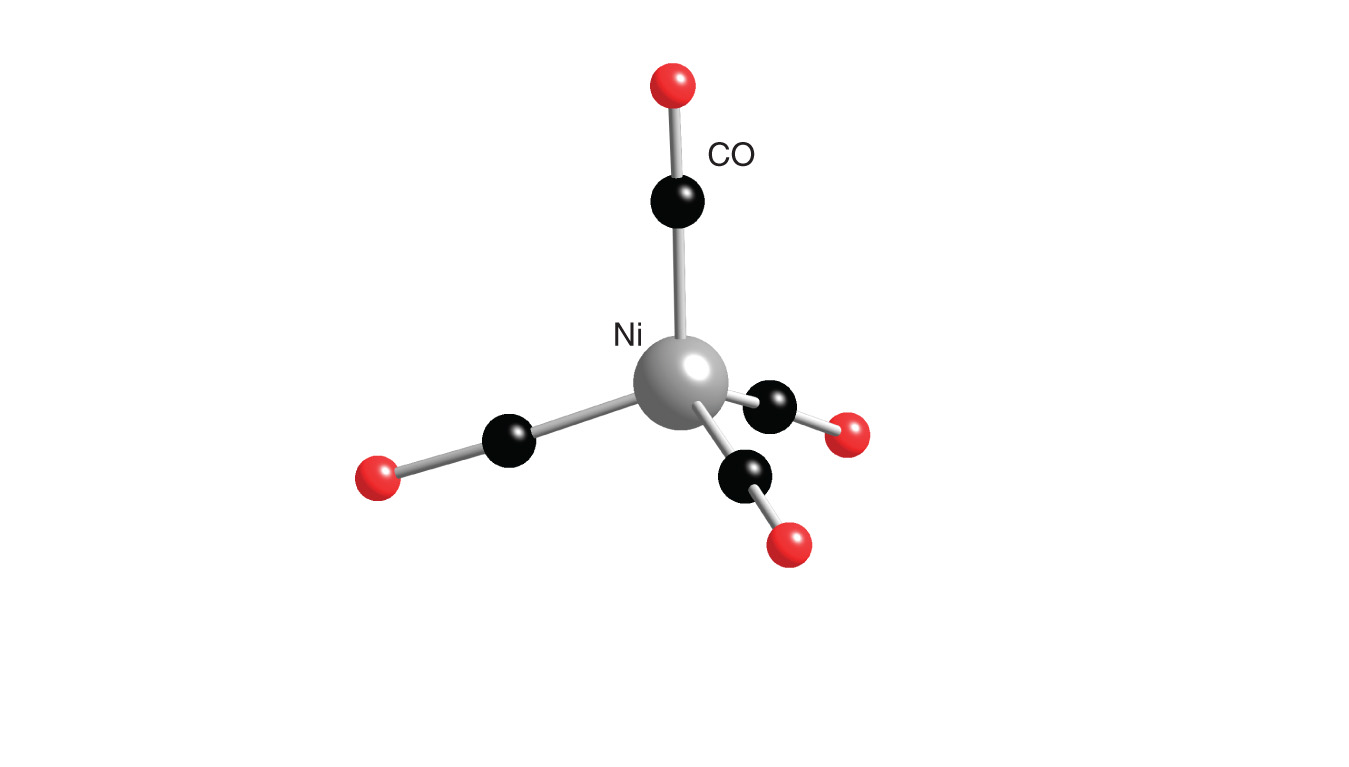
Explain bonding and geometry of Ni(CO)4 by valence bond theory (if in plus zero state it wi have its normal - Chemistry - Coordination Compounds - 14313495 | Meritnation.com
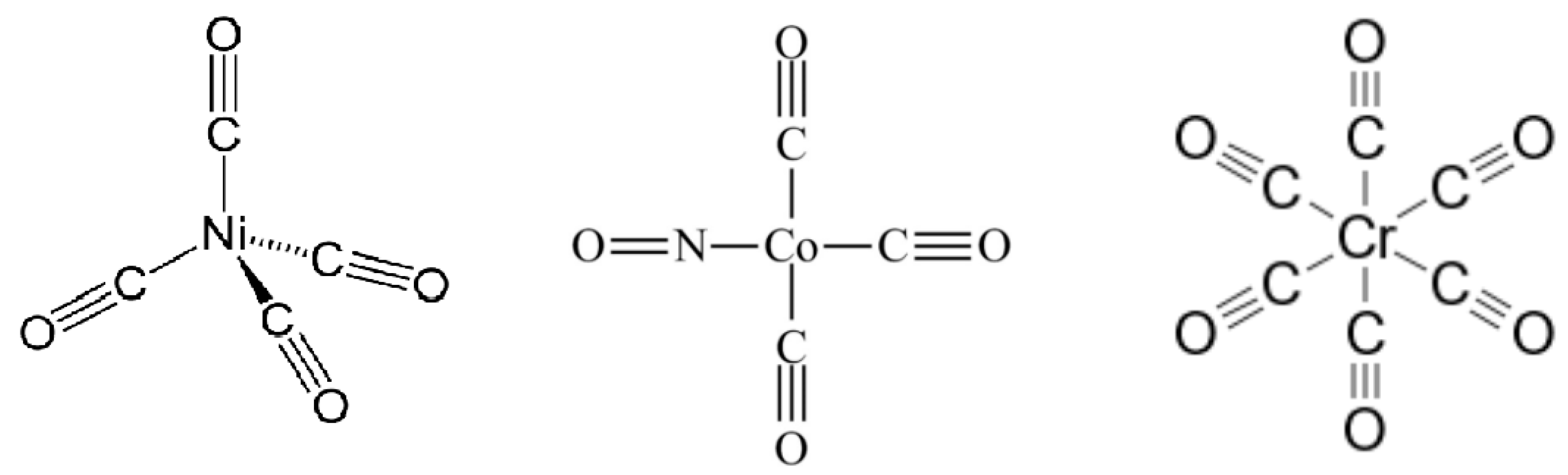
Chemistry | Free Full-Text | Dissociative Electron Attachment Cross Sections for Ni(CO)4, Co(CO)3NO, Cr(CO)6

Excited States of the Nickel Carbonyls Ni(CO) and Ni(CO)4: Challenging Molecules for Electronic Structure Theory | The Journal of Physical Chemistry A

Nickel Carbonyl, Preparation, Structure and Properties | Organometallic Chemistry | Inorganic Chem - YouTube


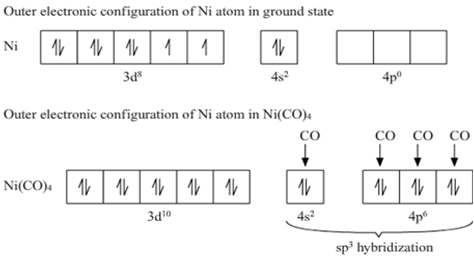
![Hybridization-Ni(CO)4 | [Ni(CN)4]2-| [Ni(Cl)4]2- | Structure-Parmagnetic-Diamagnetic-Examples-dsp2 Hybridization-Ni(CO)4 | [Ni(CN)4]2-| [Ni(Cl)4]2- | Structure-Parmagnetic-Diamagnetic-Examples-dsp2](http://www.adichemistry.com/jee/qb/coordination-chemistry/1/q1-3.png)
![Among [Ni(CO)4], [Ni(CN)4]^(2-), [NiCl4]^(2-) species, the hybridizati Among [Ni(CO)4], [Ni(CN)4]^(2-), [NiCl4]^(2-) species, the hybridizati](https://d10lpgp6xz60nq.cloudfront.net/physics_images/A2Z_CHM_XII_C09_E01_327_S01.png)
![Hybridization-Ni(CO)4 | [Ni(CN)4]2-| [Ni(Cl)4]2- | Structure-Parmagnetic-Diamagnetic-Examples-dsp2 Hybridization-Ni(CO)4 | [Ni(CN)4]2-| [Ni(Cl)4]2- | Structure-Parmagnetic-Diamagnetic-Examples-dsp2](http://www.adichemistry.com/jee/qb/coordination-chemistry/1/q1-1.png)

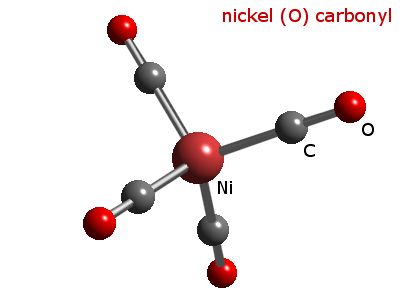



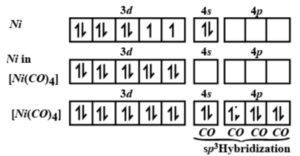

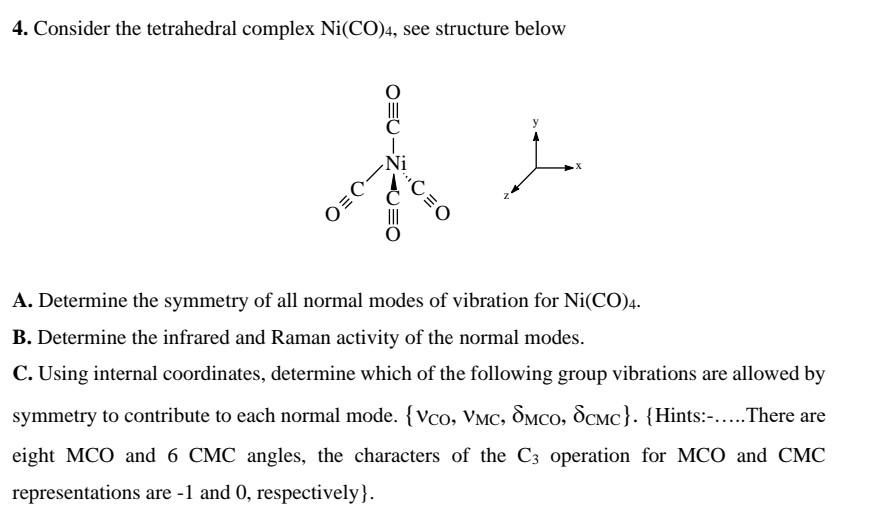
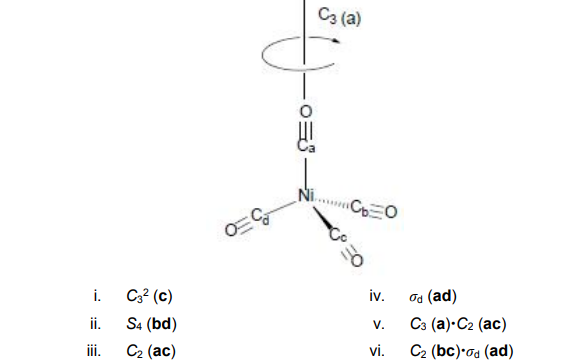
![Write the Hybridization and Magnetic Behaviour of the Complex [Ni(Co)4]. - Chemistry | Shaalaa.com Write the Hybridization and Magnetic Behaviour of the Complex [Ni(Co)4]. - Chemistry | Shaalaa.com](https://www.shaalaa.com/images/_4:0c935226f0c2451f9878137028e5360f.png)