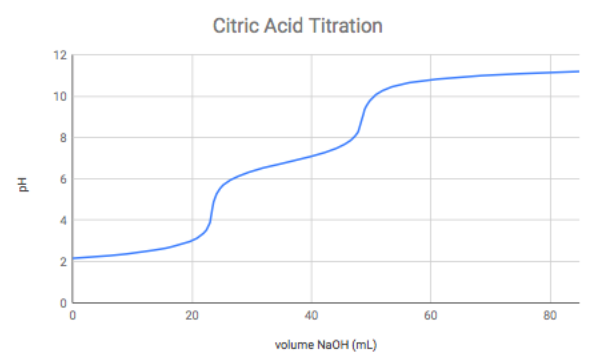
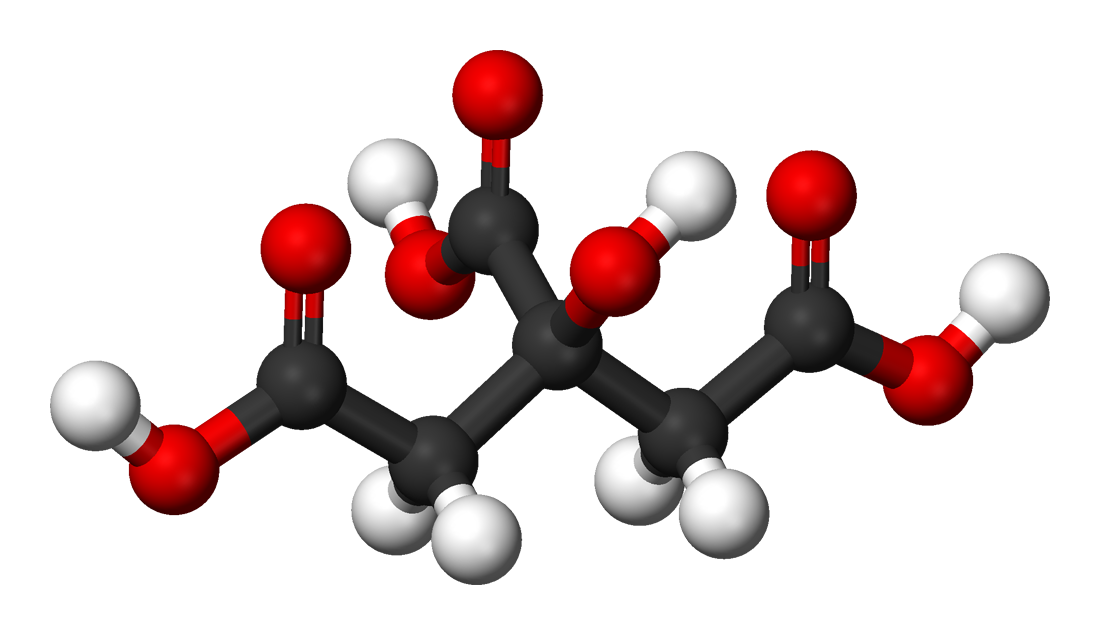
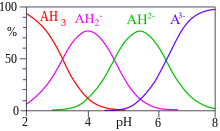
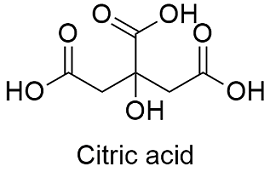
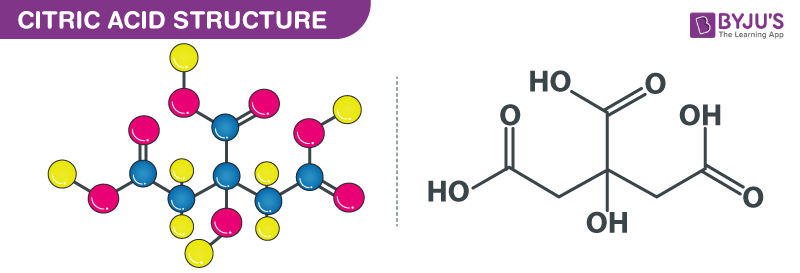
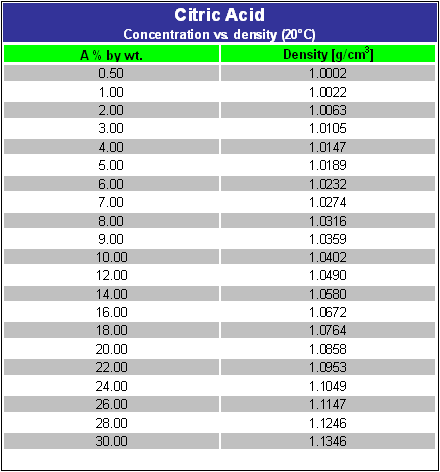
SOLVED: Citric acid has 3 pKa values: 3.128, 4.761, 6.396. Calculate the Kf for the metal-EDTA complex: Number

The Effects of Combination of Citric Acid and Microbial Phytase on the Egg Quality Characteristics in Laying Hens

SOLVED:Citric acid, which is present in citrus fruits, is a triprotic acid (Table 16.3) . (a) Calculate the pH of a 0.040M solution of citric acid. (b) Did you have to make

Lemon juice normally has a `pH` of `2`. If all the acid the lemon juice is citric acid and there are - YouTube
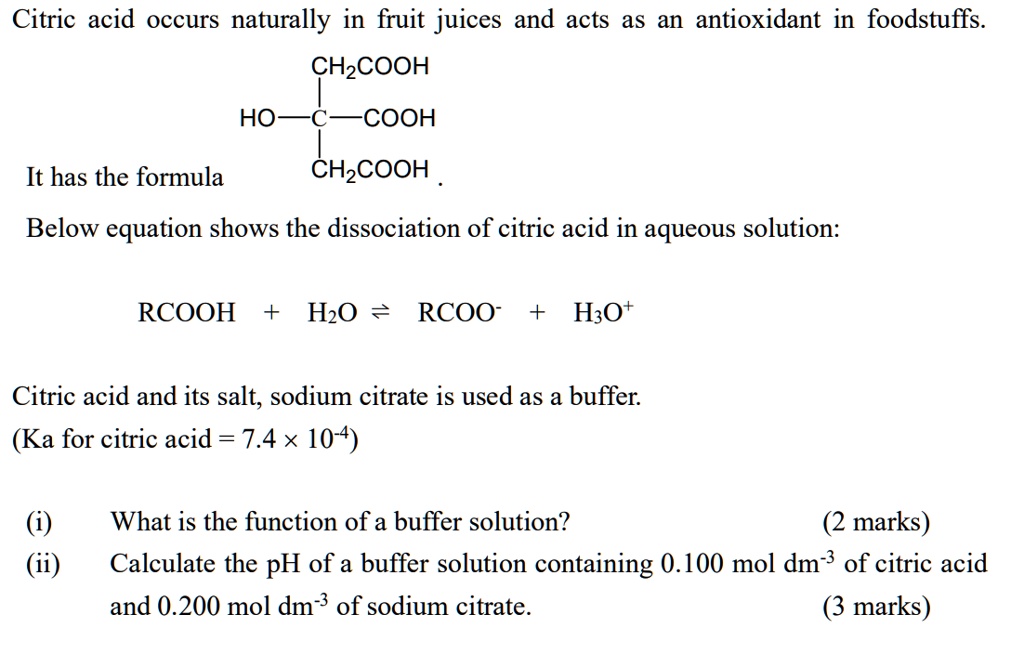
SOLVED: Citric acid occurs naturally in fruit juices and acts as an antioxidant in foodstuffs. CH2COOH HO- COOH It has the formula CHzCOOH Below equation shows the dissociation of citric acid in

Objective: To differentiate between acids and bases Do Now: List some everyday acids and bases. - ppt download
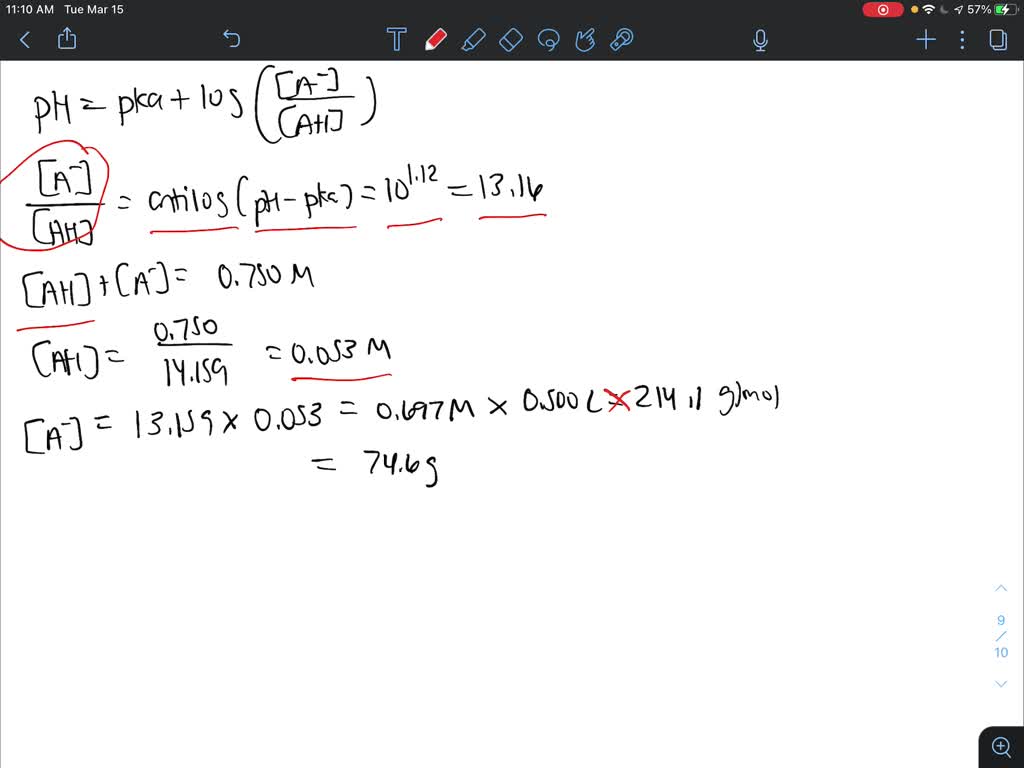
SOLVED: How will you prepare 500 ml of 0.750 M citrate buffer with pH of 4.25 from solid citric acid (C6H8O7) and solid sodium citrate (Na3C6H5O7).The Ka of citric acid is 7.40 x 10-4